What are benthic invertebrates?
Benthic invertebrates are organisms that live on the bottom of a water body (or in the sediment) and have no backbone. The size of benthic invertebrates spans 6-7 orders of magnitude1. They range from microscopic (e.g. microinvertebrates, <10 microns) to a few tens of centimetres or more in length (e.g. macroinvertebrates, >50 cm). Benthic invertebrates live either on the surface of bedforms (e.g. rock, coral or sediment – epibenthos) or within sedimentary deposits (infauna), and comprise several types of feeding groups e.g. deposit-feeders, filter-feeders, grazers and predators. The abundance, diversity, biomass and species composition of benthic invertebrates can be used as indicators of changing environmental conditions.

Photo 1. Blue Manna Crabs (Portunnus pelagicus). Photo by Tom Rose (WRC-WA)
What causes benthic invertebrate communities to change?
- The distribution and abundance of benthic invertebrate species may be profoundly influenced by a wide variety of physical parameters (e.g. substrate composition, water temperature, depth, dissolved oxygen concentrations, pH, salinity, sediment C/N ratios2 and hydrography). Spatial and temporal differences in benthic species composition may also be influenced by a range of biological factors (e.g. primary productivity, competition and acclimatisation). Natural seasonal and inter-annual changes in these variables can also modify recruitment success and mortalities of individual species, and consequently the community structure of the benthos. Such cyclical changes and often random inter-annual variation often make long-term changes in benthic communities caused by humans difficult to detect.
- Benthic communities can change in response to nutrient enrichment leading to eutrophication, and this has occurred around the world. The effects of nutrients on benthos are thought to be mainly indirect3. Nutrients may initially stimulate benthic communities because more food is supplied in the form of plant material and organic detritus3. However, as sediment organic matter increases, the oxygenated portion of the sediment can become limited to the sediment surface or be eliminated altogether, and dissolved oxygen concentrations can drop to levels that are lethal for some organisms3. Under extreme conditions organic enrichment can lead to increased periods of hypoxia or even anoxia3. Under such conditions, mobile organisms leave the affected area and sessile species die. Defaunated areas tend to be recolonised by a less diverse range of opportunisitic species tolerant of low oxygen conditions or those better at first exploiting open spaces left after all the original animals have died or migrated (e.g. small polychaete worms, nematodes and clams)3.
- Toxins produced by harmful algae can bioaccumulate to lethal levels in molluscs (shellfish), crustaceans, polychaetes and echinoderms, and cause the loss of herbivorous and predatory species4.
- Heavy metals and other toxicants derived from a range of agricultural, industrial and domestic sources can have several lethal and sub-lethal effects on benthic organisms. Under contaminated conditions, communities tend to become simplified e.g. they become dominated by fewer more tolerant species able to survive and reproduce under those conditions5. Physical abnormalities may also occur. For example, imposex in marine gastropods is caused by butyl and phenol tins found in boat antifouling paints6.
- Trawling and dredging dislodges epifauna and infauna and may result in the collection and mortality of a substantial invertebrate bycatch7. The magnitude and persistence of dredging impacts varies between species7. The loss of sensitive species can cause a change in community structure, although such changes are often hard to detect at first and can be small in comparison to natural variability measured over seasons and years78.
- Modification of river flow to estuaries by various forms of abstraction and regulation can modify the salinity9, water temperature, pH and ionic structure of the water column10 and can lower dissolved oxygen concentrations at depth9. Replacement of the existing benthic community with other benthic species may occur as a result of physiological stress and/or by competition or predation by species better physiologically suited to the modified conditions9.
- Introduced marine pests can displace indigenous species directly by predation and competitive exclusion, or indirectly by changing the physical and biological characteristics and structure of habitats i.e. their function4. Structural changes include the loss of supporting habitat such as seagrass meadows and sandy soft bottom areas. Functional changes may include altered nutrient cycling4 (including changes in denitrification rates11 and denitrification efficiencies). The effects of exotic species on benthic communities can be long-term and are often irreversible12.
- Low pH runoff from acid sulfate soils can cause mass mortalities of crustaceans and shellfish13.
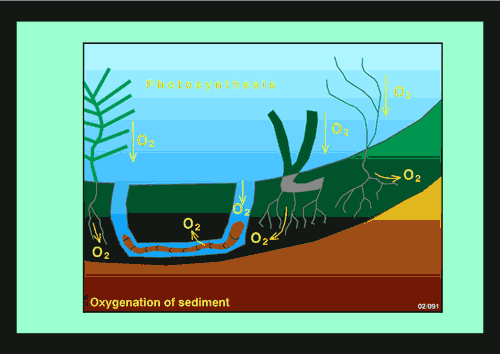
Figure 1. Burrowing and tube-building by deposit-feeding benthic invertebrates (bioturbators) helps to mix the sediment and enhances aerobic decomposition of organic matter31415. Nitrification and denitrification are also enhanced because a range of oxygenated and anoxic micro-habitats are created. For example, the area of oxic-anoxic boundaries and the surface area available for diffusive exchange is increased by tube-building invertebrates1416.
Significance of benthic invertebrates
The biomass of benthic invertebrates in estuaries and coastal embayments is often high. It declines if communities are affected by prolonged periods of poor water quality especially when anoxia and hypoxia are common14. Burrowing and tube-building by deposit-feeding benthic invertebrates (bioturbators) helps to mix the sediment and enhances decomposition of organic matter31415. Nitrification and denitrification are also enhanced because a range of oxygenated and anoxic micro-habitats are created. For example, the area of oxic-anoxic boundaries and the surface area available for diffusive exchange is increased by tube-building invertebrates1416. Loss of nitrification and denitrification (and increased ammonium efflux from sediment) in coastal and estuarine systems is an important cause of hysteresis17, which can cause a shift from clear water to a turbid state1418.
The loss of benthic suspension-feeding macroinvertebrates can further enhance turbidity levels because these organisms filter suspended particles including planktonic algae, and they enhance sedimentation rates through biodeposition (i.e. voiding of their wastes and unwanted food)3. Changes in the macrofauna (and flora) cause changes in nutrient storage pools and in the flux of nutrients between microfauna (and flora) and macrofauna and flora. Macrofauna are also important constituents of fish diets and thus are an important link for transferring energy and nutrients between trophic levels and driving pelagic fish and crustacean production. It is for these reasons and others, that benthic invertebrates are extremely important indicators of environmental change.
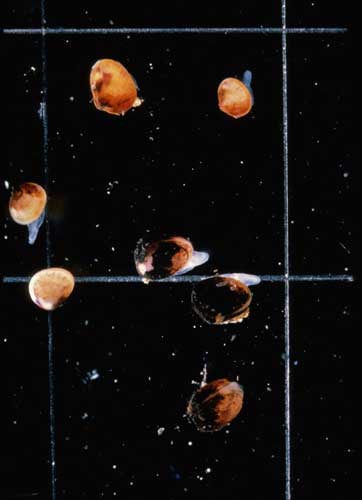
Photo 2. The micro-clam (Arthritica semen) is a small bivalve (e.g. usually 1-3 mm) that is ubiquitous in many southwestern Australian estuaries. Photo by Tom Rose (WRC-WA).
Considerations for measurement and interpretation
Measurements of change in benthic marine communities have for several decades been widely used in identifying and monitoring human impacts on the sea. Macrobenthic analyses have proven to be useful in assessing the environmental impacts of coastal discharges1920, chemical contamination of sediments2122 commercial dredging23, sludge dumping24, trawling252627 oil exploration2829 and introduced marine pests3031. This is largely because benthic organisms are relatively non-mobile and integrate effects of pollutants over time. Most importantly, however, macrobenthic organisms are comparatively easy to sample, identify and count.
Macrobenthic monitoring programs are almost always a compromise between the scientific ideal and political, financial and logistical constraints32. The costs of biological monitoring are relatively high compared to physical or chemical monitoring (largely because of the labour intensive nature of field sampling and laboratory analysis). But physical/chemical data are only an indirect measure of ecosystem health. Direct monitoring of the biota is the only way in which an unequivocal assessment of ecosystem health can be obtained.
Methods of sampling benthic invertebrate populations vary with the types of organisms under study, and the type of bottom. Many organisms that live on, rather than within, the bedforms can be captured by trawls, dredges and seine nets similar to those used by commercial fisheries. Unfortunately, most of these methods are semi-quantitative at best, and do not always provide reliable estimates of population sizes.The sampling efficiency of trawls and dredges, for example, is greatly influenced by variations in the composition and topography of the seafloor3334. Furthermore sample size is difficult to determine for trawls and dredge gear, and even harder to replicate, as vessel speed and length of tow are not easily controlled35.
Diver sampling is arguably the optimum quantitative approach to sampling large epibenthic assemblages31. The sampling efficiency of a diver-operated sled is not directly affected by undulations in the bedforms or by variations in bottom type. The sample size is predetermined and can be consistently replicated in space and time. In addition, the physical collection of biological samples facilitates accurate identifications of epibenthic species encountered and provides more precise estimates of species abundance and biomass. Unfortunately the dive survey method is labour intensive and relatively more expensive than trawling, and dredging. More importantly, it cannot be employed in deep waters (30 m) where dive time is restrictive, and in areas of high turbidity where poor visibility can strongly influence collection efficiency.
To capture smaller invertebrates that live beneath the surface of sediments, the sampling device must be capable of digging into the sediments. A large variety of corers and grab samplers have been developed for this purpose (e.g. Petersen grab, Smith-McIntyre grab, Knudsen sampler, and Barnett-Hardy corer)35. Most of these apparatus are geared at taking quantitative samples of sediments of known area and depth. Their performance, however, varies with sediment structure and depth. Many corers and grab samples, for example, are unable to sample animals inhabiting coarse sand and gravel, as they are unable to penetrate and keep these sediments when being brought to the surface of the water. Because of its ability to sample quantitatively a wide range of sediment types and a broad range of depths, the spring-loaded 0.1m2 Smith-McIntyre grab has found general acceptance among oceanographers and benthic ecologists.
There are very good arguments that have been made that indicate any changes caused by humans are only significant and important if they cause a fluctuation that is greater than the average fluctuation that occurs naturally within the population. Monitoring change in ecology is actually relatively straightforward. The real difficulties lie in interpreting the CAUSES of such changes, particularly when people try to delineate cause (e.g. human impacts), when they have only been monitoring change. The detection of cause is an experimental design issue and cannot be reached via simple monitoring. There are some very well documented approaches that now allow temporal and spatial variation to be incorporated into the experimental designs so that impacts caused by humans can be detected363738394041, in a context of a naturally variable world4142. These have been tested in a number of situations and work. Moreover, examining the entire assemblage/community tends to provide a more powerful test of whether there has been a human impact (still making use of appropriate experimental designs) than monitoring a single population, especially when the population may have been chosen because it was believed to be an “indicator”. The excellent work done in Europe (Plymouth Marine Laboratories) has shown this very clearly and even developed new statistical techniques that can be used to analyse the community data.
Existing information and data
Museums, universities and other research institutions and state governments have information on benthic invertebrates. Many museums and universities have collections that can be viewed to help in identification of benthic organisms. There are also literally hundreds of taxonomic data bases on benthic invertebrates that can be found quickly on a web search. The Australian Museum website (australianmuseum.net.au/) has many good links and is a good place to start.
More information on biota removal/disturbance.
Key questions
What are the benthic organisms in your estuary? What eats them and how important are they to the food chain or recreational fishing? What role do they play and how important are they in nutrient and energy cycling? Are they useful for monitoring your estuary or coastal area? What is the standing crop and how will this be affected by development on the water’s edge?
References
- Heip, C. 1995. Eutrophication and zoobenthos dynamics. Ophelia 41, 113-136.
- Nielson, J. and P. Jernakoff, P. 1996. A review of the interaction of sediment and water quality with benthic communities. Port Phillip Bay Environmental Study. Technical Report No. 25, 1-130.
- Williamson, A.T., Bax, N.J., Gonzalez, E. & Geeves, W. (2002) Development of a Regional Risk Management Framework for APEC Economies for Use in the Control and Prevention of Introduced Marine Pests (PDF 4.17 Mb). APEC MRC-WG Final Report, 208 pp.
- Connell, D.W. and Miller, G.J. 1984. Chemistry and Ecotoxicology of Pollution. John Wiley & Sons, Inc., pp. 444.
- Currie, D.R. and Parry, G.D. 1996. Effects of a scallop dredging on a soft sediment community: a large-scale experimental study. Marine Ecology Progress Series 134, 131-150.
- D.R. Currie, and Parry, G.D. 1999. Impacts and efficiency of scallop dredging on different soft substrates. Canadian Journal of Fisheries and Aquatic Sciences 56, 539-550.
- Pierson, W.L., Bishop, K., Van Senden, D., Horton, P.R. and Adamantidis. 2002. Environmental Water Requirements to Maintain Estuarine Processes. Report Number 3, Environmental Flows Initiative Technical Report, Commonwealth of Australia, pp. 147.
- Radke, L.C. in prep. Chemical Diversity in Southeastern Australian Saline Lakes II: Biotic Implications
- Currie, D.R. and Parry, G.D. 1999. Changes to Benthic Communities over 20 years in Port Phillip Bay, Victoria, Australia. Marine Pollution Bulletin 38(1) 36-43.
- Nixon, S.W 1988, cited in Harris 1999. Comparison of the biogeochemistry of lakes and estuaries: ecosystem processes, functional groups, hysteresis effects and interactions between macro- and microbiology. Marine and Freshwater Research 50, 791-811.
- Bird, F.L. 1994. The effects of bioturbation in Port Phillip Bay. Technical Report No.14, CSIRO Port Phillip Bay Environmental Study, Melbourne, Australia, pp. 22.
- Kristensen, E., Jensen, M.H., Aller, R.C. 1991. Direct measurement of dissolved inorganic nitrogen exchange and denitrification in individual polychaete (Nereis virens) burrows. Journal of Marine Research 49, 355-377.
- Scheffer, M. 2001. Alternative attractors of shallow lakes (PDF 0.24 Mb) . The Scientific World 1, 254-2634.
- Anderlini, V.C. and Wear, R.G. (1992). The effects of sewage and natural seasonal disturbances on benthic macrofaunal communities in Fitzroy Bay, Wellington, New Zealand. Marine Pollution Bulletin. 24, 21-26.
- Ashton P.H. and Richardson B.J. (1995). Biological monitoring of the marine ocean outfall at Black Rock, Victoria, Australia. Marine Pollution Bulletin. 31, 334-340.
- Coleman, N. (1993). The macrobenthos of Corio Bay. Environment Protection Authority. SRS 91/010, Melbourne, Australia.
- Guns M., Van Hoeyweghen P., Vyncke W. and Hillewaert H.(1999). Trace metals in selected benthic invertebrates from Belgian coastal waters (1981-1996). Marine Pollution Bulletin. 38, 1184-1193.
- Johnson L.J. and Frid C.L.J. (1995). The Recovery of benthic communities along the County Durham coast after cessation of colliery spoil dumping. Marine Pollution Bulletin. 30, 215-220.
- Jarho P., Urtti A., Jarvinen K., Pate D.W., Jarvinen T., Kenny A.J. and Rees H.L. (1996). The effects of marine gravel extraction on the macrobenthos: results 2 years post-dredging. Marine Pollution Bulletin. 32, 615-622.
- Currie, D.R. and Parry, G.D. (1996). The effects of scallop dredging on a soft-sediment community: a large scale experimental study. Marine Ecology Progress Series. 134, 131-150.
- Hill A.S., Veale L.O., Pennington D., Whyte S.G., Brand A.R. and Hartnoll R.G. (1999). Changes in Irish Sea benthos: possible effects of 40 years of dredging. Estuarine, Coastal and Shelf Science. 48, 739-750.
- Collie J.S., Hall S.J., Kaiser M.J. and Poiner I.R (2000). A quantitative analysis of fishing impacts on shelf-sea benthos. Journal of Animal Ecology. 69, 785-798.
- Gray, J.S., Clarke, K.R., Warwick, R.M. and Hobbs, G. (1990). Detection of initial effects of pollution on marine benthos: an example of the Ekofisk and Eldfisk oilfields, North Sea. Marine Ecology Progress Series. 66, 285-299.
- Kingston P.F., Dixon I.M.T., Hamilton S. and Moore D.C.(1995). The Impact of the Braer oil spill on the macrobenthic infauna of the sediments off the Shetland Islands. Marine Pollution Bulletin. 7, 445-459.
- Currie, D.R., McArthur, M.A. and Cohen, B.F. (1999a). Exotic marine pests in the port of Geelong, Victoria. pp. 227-246. In: Hewitt, C.L., Campbell, M.L., Thresher, R.E. and Martin, R.B. (eds.). Marine Biological Invasions of Port Phillip Bay, Victoria. Centre for Research on Introduced Marine Pests. Technical Report 20. CSIRO Marine Research, Hobart. 344 pp.
- Cohen, B.F., Currie, D.R. and McArthur, M.A. (2000). Epibenthic community structure in Port Phillip Bay, Victoria, Australia. Marine and Freshwater Research. 51, 689-702.
- Warwick, R.M. (1993). Environmental impact studies on marine communities: pragmatical considerations. Australian Journal of Ecology. 18, 63-80.
- Jean, F. and Hily, C. (1994). Quantitative sampling of soft-bottom macroepifauna for assessing the benthic system in the Bay of Brest, France. Oceanologica Acta 17 (3) 319-330.
- Currie, D.R. and Parry, G.D. (1999). Impacts and efficiency of scallop dredging on different soft substrates. Canadian Journal of Fisheries and Aquatic Science 56, 539-550.
- Holme, N.A. and McIntyre, A.D. (1971). ‘Methods for the study of Marine Benthos.’ (Blackwell Scientific Publications: Oxford and Edinburgh).
- Harris, G.P. 1999. Comparison of the biogeochemistry of lakes and estuaries: ecosystem processes, functional groups, hysteresis effects and interaction between macro- and microbiology. Marine and Freshwater Research 50, 791-811.
- see Cappo, M., Alongi, D.M., Williams, D, and N. Duke. 1995. A review and synthesis of Australian Fisheries Habitat Research: Major threats, issues and gaps in knowledge of coastal and marine fisheries habitats. Fisheries Research and Development Corporation.
- Sammut, J., Melville, M.D., Callinan, R.B. and Fraser, G.C. 1995. Estuarine acidification: Impacts on aquatic biota of draining acid sulphate soils. Australian Geographical Studies 33(1), 89-100.
- see page 8.1-20 of ANZECC/ARMCANZ (October 2000) Australian Guidelines for Fresh and Marine Water Quality.
- Ellis, D.V. and Pattisina, L.A. 1990. Widespread neogastropod imposex: A biological indicator of global TBT contamination. Marine Pollution Bulletin 21(5), 248-253.
- Grebmeir, J.M., McRoy, C.P., and Feder, H.M. 1988. Pelagic-benthic coupling on the shelf of the northern Bering and Chukchi Seas. I. Food supply source and benthic biomass. Marine Ecology Progress Series 48, 57-67.
- Underwood, A.J. 1991. Beyond BACI: Experimental designs for detecting human environmental impacts on temporal variations in natural populations. Australian Journal of Marine and Freshwater Research 42, 569-588.
- Underwood, A.J. 1992. Beyond BACI: the detection of environmental impacts on populations in the real, but variable world. J. Exp. Mar. Biol. Ecol. 161, 145-178.
- Underwood, A.J. 1993. The mechanics of spatially replicated sampling programmes to detect environmental impacts in a variable world. Aust. J. Ecol. 18, 99-116.
- Glasby, T.M. 1997. Analysing data from post-impact studies using asymmetrical analyses of variance: a case study of epibiota on marinas. Aust. J. Ecol. 22, 448-459.
- Underwood, A.J. 1989. The analysis of stress in natural populations. Biol. J. Linn. Soc. 37, 51-78.
- Underwood, A.J. 1995. Detection and measurement of environmental impacts. In Underwood, A.J. and Chapman, M.G. (eds), Coastal Marine Ecology of Temperate Australia, University of New South Wales Press, Sydney, Australia. pp. 311-324.
- Skilleter, G.A. 1995. Environmental Disturbances. In Underwood, A.J. and Chapman, M.G. (eds), Coastal Marine Ecology of Temperate Australia, University of New South Wales Press, Sydney, Australia. pp. 263-276.
Author
David Currie, Centre for Environmental Management, Central Queensland University
Contributors
Tom Rose, Waters and Rivers Commission, Western Australia
Greg Skilleter, Marine and Estuarine Ecology Unit, University of Queensland
- Heip, C. 1995. Eutrophication and zoobenthos dynamics. Ophelia 41, 113-136. ↩
- Grebmeir, J.M., McRoy, C.P., and Feder, H.M. 1988. Pelagic-benthic coupling on the shelf of the northern Bering and Chukchi Seas. I. Food supply source and benthic biomass. Marine Ecology Progress Series 48, 57-67. ↩
- Nielson, J. and P. Jernakoff, P. 1996. A review of the interaction of sediment and water quality with benthic communities. Port Phillip Bay Environmental Study. Technical Report No. 25, 1-130. ↩ ↩ ↩ ↩ ↩ ↩ ↩ ↩
- Williamson, A.T., Bax, N.J., Gonzalez, E. & Geeves, W. (2002) Development of a Regional Risk Management Framework for APEC Economies for Use in the Control and Prevention of Introduced Marine Pests (PDF 4.17 Mb). APEC MRC-WG Final Report, 208 pp. ↩ ↩ ↩
- Connell, D.W. and Miller, G.J. 1984. Chemistry and Ecotoxicology of Pollution. John Wiley & Sons, Inc., pp. 444. ↩
- see page 8.1-20 of ANZECC/ARMCANZ (October 2000) Australian Guidelines for Fresh and Marine Water Quality. ↩
- Currie, D.R. and Parry, G.D. 1996. Effects of a scallop dredging on a soft sediment community: a large-scale experimental study. Marine Ecology Progress Series 134, 131-150. ↩ ↩ ↩
- D.R. Currie, and Parry, G.D. 1999. Impacts and efficiency of scallop dredging on different soft substrates. Canadian Journal of Fisheries and Aquatic Sciences 56, 539-550. ↩
- Pierson, W.L., Bishop, K., Van Senden, D., Horton, P.R. and Adamantidis. 2002. Environmental Water Requirements to Maintain Estuarine Processes. Report Number 3, Environmental Flows Initiative Technical Report, Commonwealth of Australia, pp. 147. ↩ ↩ ↩
- Radke, L.C. in prep. Chemical Diversity in Southeastern Australian Saline Lakes II: Biotic Implications ↩
- see Cappo, M., Alongi, D.M., Williams, D, and N. Duke. 1995. A review and synthesis of Australian Fisheries Habitat Research: Major threats, issues and gaps in knowledge of coastal and marine fisheries habitats. Fisheries Research and Development Corporation. ↩
- Currie, D.R. and Parry, G.D. 1999. Changes to Benthic Communities over 20 years in Port Phillip Bay, Victoria, Australia. Marine Pollution Bulletin 38(1) 36-43. ↩
- Sammut, J., Melville, M.D., Callinan, R.B. and Fraser, G.C. 1995. Estuarine acidification: Impacts on aquatic biota of draining acid sulphate soils. Australian Geographical Studies 33(1), 89-100. ↩
- Nixon, S.W 1988, cited in Harris 1999. Comparison of the biogeochemistry of lakes and estuaries: ecosystem processes, functional groups, hysteresis effects and interactions between macro- and microbiology. Marine and Freshwater Research 50, 791-811. ↩ ↩ ↩ ↩ ↩ ↩
- Bird, F.L. 1994. The effects of bioturbation in Port Phillip Bay. Technical Report No.14, CSIRO Port Phillip Bay Environmental Study, Melbourne, Australia, pp. 22. ↩ ↩
- Kristensen, E., Jensen, M.H., Aller, R.C. 1991. Direct measurement of dissolved inorganic nitrogen exchange and denitrification in individual polychaete (Nereis virens) burrows. Journal of Marine Research 49, 355-377. ↩ ↩
- Harris, G.P. 1999. Comparison of the biogeochemistry of lakes and estuaries: ecosystem processes, functional groups, hysteresis effects and interaction between macro- and microbiology. Marine and Freshwater Research 50, 791-811. ↩
- Scheffer, M. 2001. Alternative attractors of shallow lakes (PDF 0.24 Mb) . The Scientific World 1, 254-2634. ↩
- Anderlini, V.C. and Wear, R.G. (1992). The effects of sewage and natural seasonal disturbances on benthic macrofaunal communities in Fitzroy Bay, Wellington, New Zealand. Marine Pollution Bulletin. 24, 21-26. ↩
- Ashton P.H. and Richardson B.J. (1995). Biological monitoring of the marine ocean outfall at Black Rock, Victoria, Australia. Marine Pollution Bulletin. 31, 334-340. ↩
- Coleman, N. (1993). The macrobenthos of Corio Bay. Environment Protection Authority. SRS 91/010, Melbourne, Australia. ↩
- Guns M., Van Hoeyweghen P., Vyncke W. and Hillewaert H.(1999). Trace metals in selected benthic invertebrates from Belgian coastal waters (1981-1996). Marine Pollution Bulletin. 38, 1184-1193. ↩
- Johnson L.J. and Frid C.L.J. (1995). The Recovery of benthic communities along the County Durham coast after cessation of colliery spoil dumping. Marine Pollution Bulletin. 30, 215-220. ↩
- Jarho P., Urtti A., Jarvinen K., Pate D.W., Jarvinen T., Kenny A.J. and Rees H.L. (1996). The effects of marine gravel extraction on the macrobenthos: results 2 years post-dredging. Marine Pollution Bulletin. 32, 615-622. ↩
- Currie, D.R. and Parry, G.D. (1996). The effects of scallop dredging on a soft-sediment community: a large scale experimental study. Marine Ecology Progress Series. 134, 131-150. ↩
- Hill A.S., Veale L.O., Pennington D., Whyte S.G., Brand A.R. and Hartnoll R.G. (1999). Changes in Irish Sea benthos: possible effects of 40 years of dredging. Estuarine, Coastal and Shelf Science. 48, 739-750. ↩
- Collie J.S., Hall S.J., Kaiser M.J. and Poiner I.R (2000). A quantitative analysis of fishing impacts on shelf-sea benthos. Journal of Animal Ecology. 69, 785-798. ↩
- Gray, J.S., Clarke, K.R., Warwick, R.M. and Hobbs, G. (1990). Detection of initial effects of pollution on marine benthos: an example of the Ekofisk and Eldfisk oilfields, North Sea. Marine Ecology Progress Series. 66, 285-299. ↩
- Kingston P.F., Dixon I.M.T., Hamilton S. and Moore D.C.(1995). The Impact of the Braer oil spill on the macrobenthic infauna of the sediments off the Shetland Islands. Marine Pollution Bulletin. 7, 445-459. ↩
- Currie, D.R., McArthur, M.A. and Cohen, B.F. (1999a). Exotic marine pests in the port of Geelong, Victoria. pp. 227-246. In: Hewitt, C.L., Campbell, M.L., Thresher, R.E. and Martin, R.B. (eds.). Marine Biological Invasions of Port Phillip Bay, Victoria. Centre for Research on Introduced Marine Pests. Technical Report 20. CSIRO Marine Research, Hobart. 344 pp. ↩
- Cohen, B.F., Currie, D.R. and McArthur, M.A. (2000). Epibenthic community structure in Port Phillip Bay, Victoria, Australia. Marine and Freshwater Research. 51, 689-702. ↩ ↩
- Warwick, R.M. (1993). Environmental impact studies on marine communities: pragmatical considerations. Australian Journal of Ecology. 18, 63-80. ↩
- Jean, F. and Hily, C. (1994). Quantitative sampling of soft-bottom macroepifauna for assessing the benthic system in the Bay of Brest, France. Oceanologica Acta 17 (3) 319-330. ↩
- Currie, D.R. and Parry, G.D. (1999). Impacts and efficiency of scallop dredging on different soft substrates. Canadian Journal of Fisheries and Aquatic Science 56, 539-550. ↩
- Holme, N.A. and McIntyre, A.D. (1971). ‘Methods for the study of Marine Benthos.’ (Blackwell Scientific Publications: Oxford and Edinburgh). ↩ ↩
- Underwood, A.J. 1991. Beyond BACI: Experimental designs for detecting human environmental impacts on temporal variations in natural populations. Australian Journal of Marine and Freshwater Research 42, 569-588. ↩
- Underwood, A.J. 1992. Beyond BACI: the detection of environmental impacts on populations in the real, but variable world. J. Exp. Mar. Biol. Ecol. 161, 145-178. ↩
- Underwood, A.J. 1993. The mechanics of spatially replicated sampling programmes to detect environmental impacts in a variable world. Aust. J. Ecol. 18, 99-116. ↩
- Glasby, T.M. 1997. Analysing data from post-impact studies using asymmetrical analyses of variance: a case study of epibiota on marinas. Aust. J. Ecol. 22, 448-459. ↩
- Underwood, A.J. 1989. The analysis of stress in natural populations. Biol. J. Linn. Soc. 37, 51-78. ↩
- Underwood, A.J. 1995. Detection and measurement of environmental impacts. In Underwood, A.J. and Chapman, M.G. (eds), Coastal Marine Ecology of Temperate Australia, University of New South Wales Press, Sydney, Australia. pp. 311-324. ↩ ↩
- Skilleter, G.A. 1995. Environmental Disturbances. In Underwood, A.J. and Chapman, M.G. (eds), Coastal Marine Ecology of Temperate Australia, University of New South Wales Press, Sydney, Australia. pp. 263-276. ↩