Acid sulfate soils (ASS) are soils and other soft sediments that contain iron sulfides (mostly pyrite (FeS2; photo 1) with typically smaller quantities of iron monosulfides (FeS)). If the iron sulfides are exposed to atmospheric oxygen they can be oxidised. Pyrite oxidation produces a cocktail of sulfuric acid, aluminum, iron and other heavy metals that can move into coastal waterways, and this can have significant environmental and economic impacts.

Photo 1. Cluster of pyrite surrounded by framboids from the Pimpama River (SE Qld) pyritic sediments2 (Photo: Micaela Preda)
The ASS of most concern have mostly developed during the Holocene period of high sea level. Between 18,000 and 6,500 years ago, sea level rose from 120 m lower than today to approximately its present position. Extensive, very shallow coastal waterways formed as the sea reached approximately its present level. During the last 6,500 years these shallow coastal waters have been infilled with coastal sediments, forming coastal plains that today extend up to tens of kilometres inland from the coast (Figure 1).
Sediments deposited in these environments can be high in sulfides such as pyrite, providing that certain conditions are met. Pyrite is the end product in a chain of reactions that require anoxic conditions, and sources of sulfur, iron and organic material. The first step is promoted by sulfate-reducing bacteria and involves the reduction of sulfate from seawater to form hydrogen sulfide gas. This reduced form of sulfur can then react with the available iron to form pyrite (Photo 1 and Figure 2). As a consequence of these conditions prevailing in the Holocene period, many of our low-lying coastal plains now form tracts of ASS.
Similar sediments laid down in previous interglacial high sea level stands (e.g. Swan Valley Coastal Plain) are also potential acid sulfate soils (PASS)3. Sulfide-rich sediments continue to form today in tidal flats, salt marshes, mangroves and other coastal wetlands. Australia has roughly 50,000 km2 of ASS containing in excess of a billion tonnes of iron sulfides4.
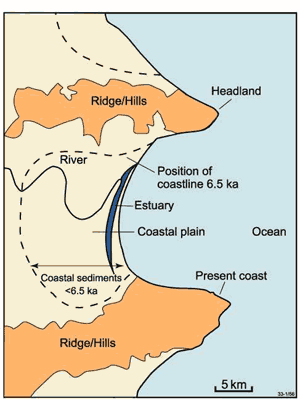
Figure 1. A map of a typical depositional coast. The dashed line indicates the mid-Holocene shoreline. The shoreline has extended seawards (prograded) due to coastal sedimentation during the last ~6,500 years of high, relatively stable sea level.
Some potential impacts of disturbed acid sulfate soils
Some known adverse impacts of ASS in coastal lowlands:
- poor water quality (e.g. dissolved metal contaminants, low pH levels and anoxic and hypoxic events);
- fish kills and pathogens in fish assemblages 5;
- loss of critical habitat areas, aquaculture production, fish stocks, wetland biodiversity and amenity5;
- acid erosion of infrastructure5; and
- the need for rehabilitation of disturbed areas5.
The public health implications of disturbing acid sulfate soils are not well understood. However, acidified coastal wetlands may provide predator-free habitat for species of mosquito that transmit arboviruses (e.g. Ross River Fever)6. Acid dust mobilised during ploughing and construction activities may also cause dermatitis and eye irritation6.
Human activities that give rise to acid sulfate runoff
Iron sulfides are stable under oxygen-free (typically waterlogged) conditions. However, the disturbance of ASS for agriculture, urban development, flood mitigation or other land uses can expose iron sulfides to air, causing them to oxidise and produce sulfuric acid. Coastal waterways with rivers in acid hazard zones are most at risk of being polluted by acid sulfate drainage.
Sulfide-bearing dredge materials deposited adjacent to open waters are also a potential source of acid drainage7.
Relevant biophysical indicators
The following changes in biophysical parameters may indicate that a coastal waterway is affected by acid leaching from pyritic sediments:
- low water column pH levels (e.g. <4 in immediate area of impact);
- a dramatic reduction in water column dissolved oxygen concentrations. Monosulfidic black oozes (MBOs) in particular can cause rapid and severe anoxic and hypoxic events8;
- excess of sulfate in the water column – chloride/sulfate ratios are often <3 in acid affected streams9, whereas chloride/sulfate ratios of seawater in a dilution series with rainwater range from ~5 to 7 (Figure 2);
- iron staining in coastal tributaries and increased dissolved aluminium, iron and potentially arsenic concentrations3 (see Figure 3 below for iron speciation);
- extremely clear water where all sediments have settled out due to the flocculating ability of aluminium (see photo 2 below);
- red lesions on fish caused by the ulcer-causing fungus of epizootic ulcerative syndrome (‘red-spot’ disease) 10;
- increased incidence of fish kills;
- the presence of acid tolerant vegetation. Acid tolerant water plant species belong mainly to the genera Nymphaea and Eleocharis11. Species from this genera can complete lifecycles at pH levels less than 3 without any apparent negative impact. The introduced Cape waterlily (Nymphaea caerulea spp zanzibarensis) grows profusely at pH less than 3 in clarified aluminium-rich waters.The native waterlily (Nymphaea gigantea) is also acid tolerant but less competitive and prolific. Native spike rushes (Eleocharis spp) are also acid tolerant and occur along much of coastal Australia where ASS have been disturbed11;
- the presence of acid tolerant fish species. The introduced and noxious mosquito fish (Gambusia holbrooki) has acid tolerant populations in the Richmond River, NSW11; and
- a longer term reduction of fish and benthic invertebrate populations.
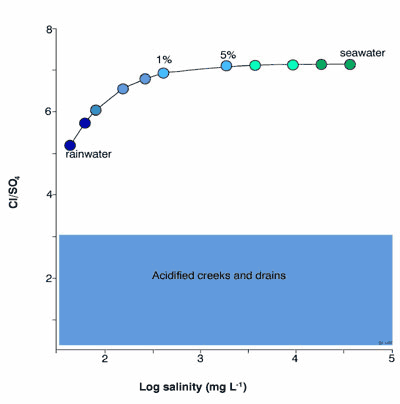
Figure 2. Chloride/sulfate ratios vs. log salinity for seawater, seawater diluted with increasing amount of precipitation, and acidified creeks and drains. The chloride/sulfate ratios of acidified creeks and streams are much lower than those of seawater, precipitation (rainwater + dust) and seawater diluted with precipitation 9. The seawater dilution model is from Radke (2000) 12.
.

Photo 2. This pond in southeast Queensland is typical of acid sulfate soil disturbance. The blue-green colour and clarity is an indicator of the presence of aluminium. The high levels of aluminium cause suspended particles in the water to clump together and drop to the bottom, resulting in clear water. The acidity of water like this is often too high (pH less than 4) to support diverse aquatic life. Note also the iron staining on the banks of the pond. Not all clear dams have high aluminium, however, when murky water goes clear over a short period of time following soil disturbance, it is a good indicator that acid sulfate soils have been exposed.(‘QASSIT, Qld Department of Natural Resources and Mines’).
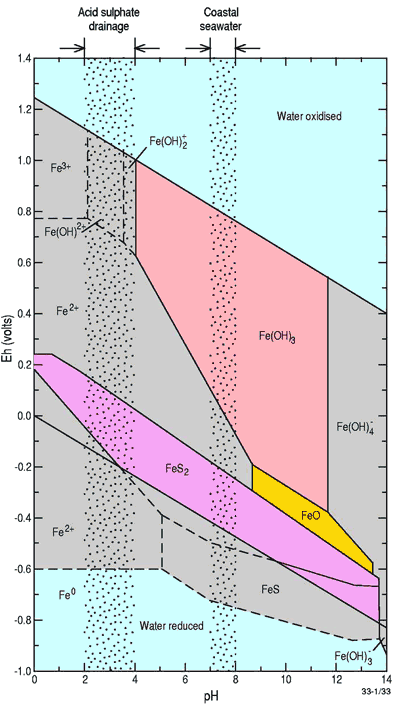
Figure 3. Stability field diagram for dissolved and solid forms of iron as a function of pH and Eh at 1 atm and 25oC. Note that more iron is in a dissolved form (Fe2+) at the pH of acid sulfate drainage than the pH of seawater. (from Elder, J.F. 1988)13. Reproduced with permission of J.F. Elder.
More information
- the Coastal Acid Sulfate Soils Program website at The Department of Environment and Heritage ;
- the NSW Government. Office of Environment & Heritage – Acid Sulfate Soils;
- the Acid Sulfate Soils Website at Queensland. Department of Natural Resources, Mines & Energy.
- the National Strategy for the Management of Coastal Acid Sulfate Soils website prepared by the National Working Party on Acid Sulfate Soils on behalf of the Standing Committee on Agriculture and Resource Management and the Agriculture and Resource Management Council of Australia and New Zealand.
- Soil and Water Processes in Acid Sulfate, Saline and Sodic Environments;
- Department of Natural Resources.
More information on pH (changed from natural).
Contributors
Brendan Brooke, Geoscience Australia
Bernie Powell, Queensland Department of Natural Resources and Mines
Micaela Preda, Queensland University of Technology
Acid sulfate soils (ASS) are soils and other soft sediments that contain iron sulfides (mostly pyrite (FeS2; photo 1) with typically smaller quantities of iron monosulfides (FeS ↩
- Preda, M and Cox M. E., 1998. Chemical character of a potential acid forming sediment, Pimpama River. In: Tibbetts I. R., Hall N. J. and Dennison W. C. (eds). Moreton Bay and Catchment, School of Marine Sciences, The University of Queensland, Brisbane, pp. 247-250. ↩
- Appleyard, S., Wong, S., Ralston, A., Angeloni, J., and Watkins, R. 2002. Groundwater acidity and arsenic contamination caused by urban development on acid sulfate soils in Perth, Western Australia. In Sustainable Managment of Acid Sulfate Soils, 5th International Acid Sulfate Soils Conference, Twin Towns Services Club, Tweed Heads, NSW Australia, pp. 69-70. ↩ ↩
- Fitzpatrick, R., Merry, R., Williams, J., White, I., Bowman, G., and G. Taylor, 2001. ↩
- Australian State of the Environment Committee. 2001. Coasts and Oceans: State of the environment Report 2001. CSIRO Publishing. Collingwood. ↩ ↩ ↩ ↩
- Powell, B. and C.R. Ahern. 1999. Queensland Government. Acid Sulfate Soils Management., pp16. ↩ ↩
- Demas, S.Y., Hall, A.M., Fanning, D.S., Rabenhorst, M.C. and E.K. Dzantor 2002. Acid sulfate soils in dredged materials from tidal Pocomoke Sound in Somerset County, MD, USA. In Sustainable Managment of Acid Sulfate Soils, 5th International Acid Sulfate Soils Conference, Twin Towns Services Club, Tweed Heads, NSW Australia, p. 8-9. ↩
- Sullivan, L.A. and Bush, R.T. 2002. Chemical behaviour of monosulfidic black oozes (MBOs) in waters: pH and dissolved oxygen. In Sustainable Managment of Acid Sulfate Soils, 5th International Acid Sulfate Soils Conference, Twin Towns Services Club, Tweed Heads, NSW Australia, p. 14-15. ↩
- Sammut, J., White, I and M.D. Melville. 1996. Acidification of an estuarine tributary in eastern Australia due to drainage of acid sulfate soils. Marine and Freshwater Research 47, 669-684. ↩ ↩
- Sammut J., Callinan R. B. & Fraser G. C. 1993. The impact of acidified water on freshwater and estuarine fish populations in acid sulfate soil environments. Proceedings of the National Conference on Acid Sulphate Soils, 24-25 June 1993, Coolangatta, 26-40. ↩
- Sammut, J, Callinan, R.B. and Fraser, G.C. (1996) An overview of the ecological impacts of acid sulfate soils in Australia. In Proceedings 2nd National Conference of Acid Sulfate Soils Robert J Smith and Associates and ASSMAC, Australia. ↩ ↩ ↩
- Radke, L.C. 2000. Solute divides and chemical facies in southeastern Australian salt lakes and the response of ostracods in time and space, Ph.D Thesis, Department of Geology, The Australian National University, pp. 232 ↩
- Elder, J.F. 1988. Metal Biogeochemistry in Surface-Water Systems – A Review of Principles and Concepts. U.S. Geological Survey Circular 1013. ↩